Image input¶
Once you have started BacStalk the Image input-tab is displayed.
BacStalk can handle either individual non-related files or an explicit file series (jpeg or tif files). The individual filenames are parsed to assign metadata (Time, Position, …) and channel information to each image in BacStalk.
Stalk-detection works best on phase contrast images.
The typical workflow involes:
Add images files to BacStalk
Assign channel information
Assign metadata
Enter pixel spacing
Select type of cells
Add files: To start, press Add files to select all files associated with an experiment. Hold Ctr to select multiple files. The selected files appear in the listbox on the left.
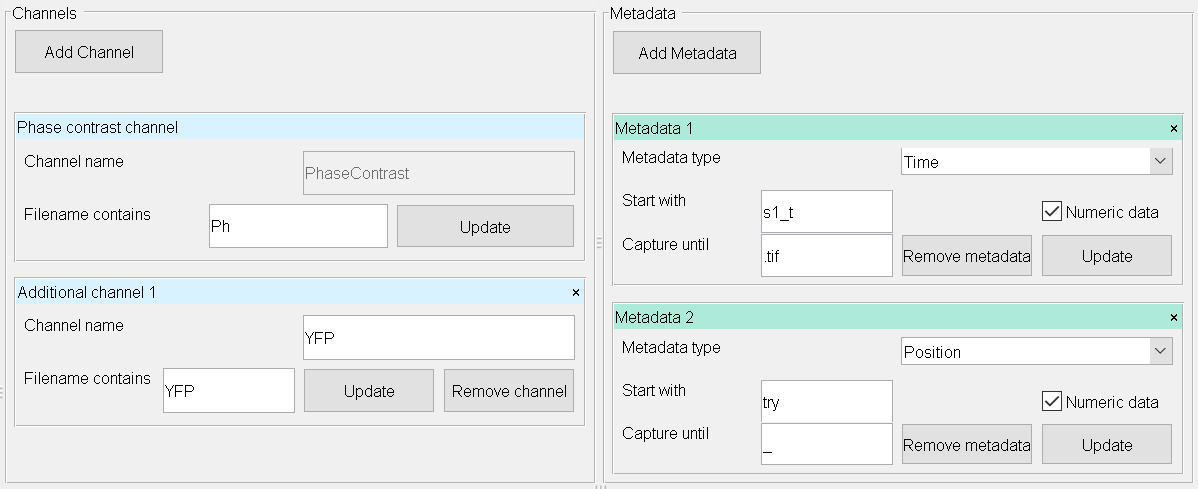
- Channels: BacStalk assigns microscope channel information to the added files by searching their filenames for key words.
Add as many channels as present in your data by clicking Add channel.
Name the channels according to your needs.
For Filename contains enter an unique identifier for the corresponding channel.
Click Update to update your file list below.
- Metadata: Assign information ( Time, Position, or Custom ).
Add as many metadata labels as present in your filenames by clicking Add metadata.
Select the metadata type.
Start with: Enter unique character(s) before the the metadata string.
Capture until: Enter the character(s) which are terminating your metadata field.
Whenever your metadata is numeric, check Numeric data.
Click Update to update your file list below.
Once the metadata field Time is used and Numeric data is enabled, BacStalk will treat the image input as time series. Drift correction can be enabled to remove a global lateral drift of the image information. In addition a time series is required for cell tracking.
Enter the image pixel size at Scaling (microns/pixel).
If your cells grow stalks, check My cells… … have stalks. If they devide via stalked budding, check My cells… … form buds. .
Example¶
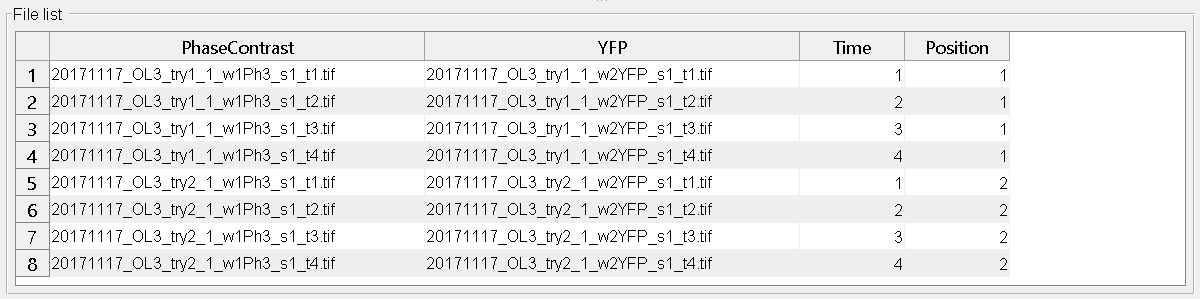
Consider the following timeseries with two positions and two channels (Phase contrast + YFP)
Files |
|---|
20171117_OL3_try1_1_w1Ph3_s1_t1.tif |
20171117_OL3_try1_1_w1Ph3_s1_t2.tif |
20171117_OL3_try1_1_w1Ph3_s1_t3.tif |
20171117_OL3_try1_1_w1Ph3_s1_t4.tif |
20171117_OL3_try1_1_w2YFP_s1_t1.tif |
20171117_OL3_try1_1_w2YFP_s1_t2.tif |
20171117_OL3_try1_1_w2YFP_s1_t3.tif |
20171117_OL3_try1_1_w2YFP_s1_t4.tif |
20171117_OL3_try2_1_w1Ph3_s1_t1.tif |
20171117_OL3_try2_1_w1Ph3_s1_t2.tif |
20171117_OL3_try2_1_w1Ph3_s1_t3.tif |
20171117_OL3_try2_1_w1Ph3_s1_t4.tif |
20171117_OL3_try2_1_w2YFP_s1_t1.tif |
20171117_OL3_try2_1_w2YFP_s1_t2.tif |
20171117_OL3_try2_1_w2YFP_s1_t3.tif |
20171117_OL3_try2_1_w2YFP_s1_t4.tif |
Enter the following channels/metadata information:
After clicking any Update-Button, the file list should look like this:
BacStalk expects monochromatic (grayscale) images. If RGB images are provided, they are displayed in colors inside BacStalk but are converted to grayscale images during processing!
Additional parameters¶
Drift correction: Enable image drift correction by image registration (requires a time series). See: 1
Scaling: Pixel size in micrometers. Required to calculate cell sizes in micrometers.
My cells…
…have stalks: Enable/disable stalk detection. Stalk detection parameters at the cell/stalk detection panel (see Data analysis) are only available if stalk detection is enabled.
…form buds: Enable/disable bud assignment.
Working with pre-segmented data from other software tools¶
If you prefer/need to use a segmentation algorithm that differs from BacStalk’s segmentation, you can import a custom segmentation from another software tool (e.g. MicrobeJ, Oufti) into BacStalk, and then proceed with the BacStalk analysis based on your custom segmentation input. Generally, if you want to use BacStalk’s analysis and visualization capabilities with custom pre-segmented data the following workflow can be used.
In the software tool that you want to use for segmentation (e.g. Oufti, MicrobeJ), export the segmented data as binary image masks (with separate images for cells and stalks).
Treat the binary masks as regular input images inside BacStalk and check Use binary masks in the Advanced options tab. Then perform the BacStalk segmentation again on these binary masks. Now BacStalk will recognize these masks.
References:
- 1
Manuel Guizar-Sicairos, Samuel T. Thurman, and James R. Fienup, “Efficient subpixel image registration algorithms,” Opt. Lett. 33, 156-158 (2008)